Articles from GenSight Biologics S.A.

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · April 8, 2025

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · February 27, 2025

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · January 15, 2025

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · December 18, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · November 13, 2024
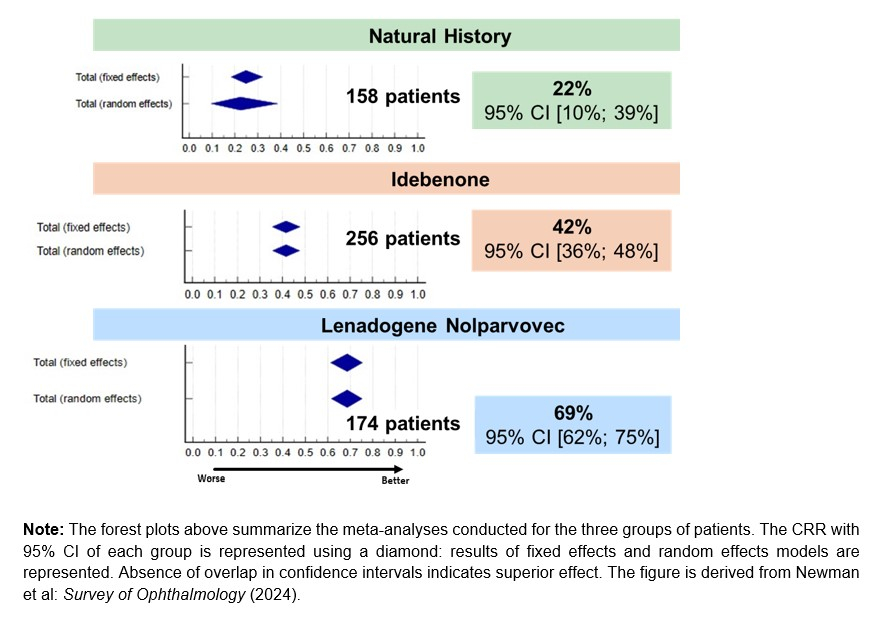
Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · October 28, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · October 24, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · September 23, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · July 23, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · June 20, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · April 4, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 22, 2024

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 12, 2024
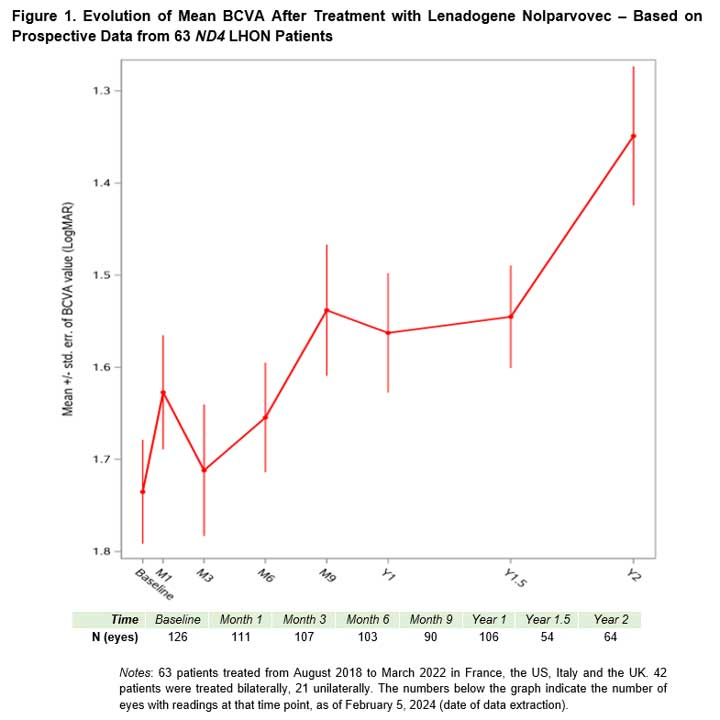
Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 6, 2024
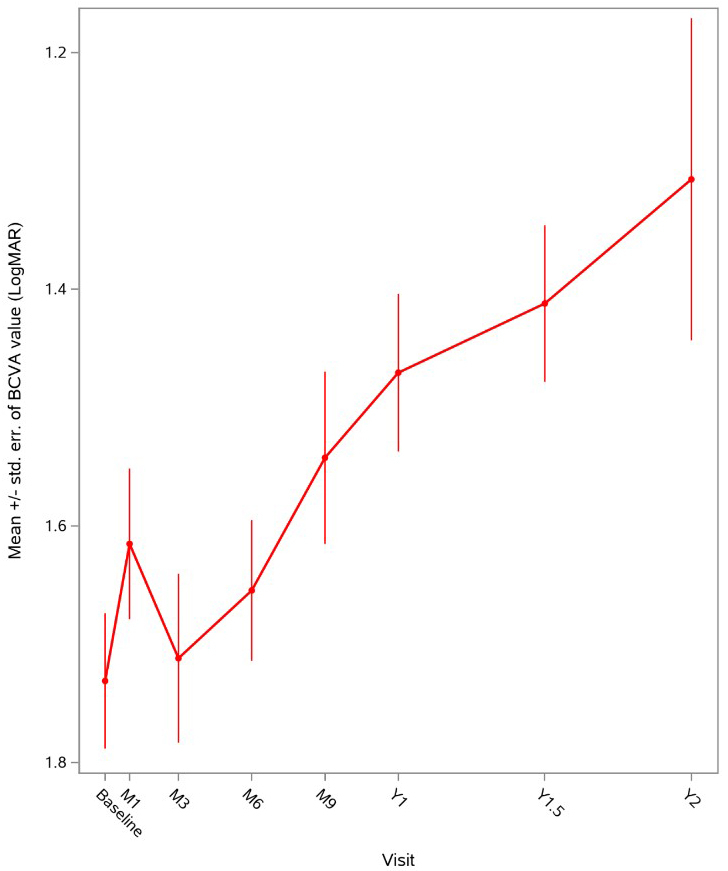
Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 15, 2023

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 13, 2023

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · March 9, 2023

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · May 19, 2022
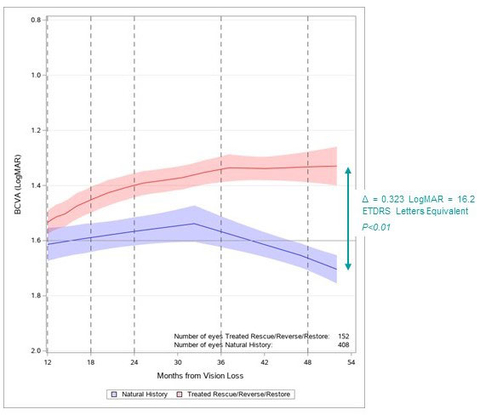
Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · January 24, 2022

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · December 14, 2021

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · November 30, 2021

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · October 6, 2021

Regulatory News:
By GenSight Biologics S.A. · Via Business Wire · September 9, 2021
