Articles from Medidata

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced that ICON plc, (NASDAQ: ICLR) a world-leading clinical research organization (CRO) powered by healthcare intelligence, will immediately begin utilizing Medidata Clinical Data Studio on its studies, differentiating ICON as the first large CRO to fully infuse this technology into its clinical workflows.
By Medidata · Via Business Wire · March 20, 2025

Medidata, a Dassault Systèmes brand, reaffirms its vision and commitment to advancing the life sciences industry and transforming patient experiences across the entire clinical development process, from pre-trial planning, to post-trial outcomes, and ongoing patient care. With advanced technologies, such as AI and virtual twins, Medidata enables biopharmaceutical companies, researchers, and patients to accelerate therapy development and improve patient lives.
By Medidata · Via Business Wire · February 4, 2025

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, has extended its 13-year relationship with Tigermed, a leading global provider of clinical research solutions across the full life cycle of global biopharmaceutical and medical device products. This extended partnership will further harness the Medidata Platform from early-phase trials to post-marketing surveillance, to optimize study workflows, ensure regulatory compliance, and improve the speed of delivering new therapies to patients worldwide, including the emerging markets.
By Medidata · Via Business Wire · January 23, 2025

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, has named Alicia Staley as chief patient officer and senior vice president, social impact and engagement, Lisa Moneymaker as senior vice president, strategic customer engagement, and Chris King as senior vice president, head of customer success, services, and support. In their roles, they will prioritize patients and customers by addressing their needs with empathy, expertise, and forward-thinking solutions. By championing these values, they will not only enhance the patient and customer experience but also propel forward Medidata’s mission to power smarter treatments and foster healthier people.
By Medidata · Via Business Wire · January 15, 2025

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, has renewed its long-standing enterprise partnership with ClinChoice, a global contract research organization (CRO). Under this new agreement, ClinChoice will utilize the Medidata Platform to streamline study data and supply management, boost trial efficiency, and accelerate growth as a full-service CRO in Asia, Europe, and North America. ClinChoice also plans to prioritize Clinical Data Studio accreditation, further enhancing its capabilities through a transformative AI-powered data quality management experience.
By Medidata · Via Business Wire · December 18, 2024

Medidata, a Dassault Systèmes brand and the leading provider of clinical trial solutions to the life sciences industry, has been recognized as a leader in Everest Group's first-ever Life Sciences Clinical Trial Management System Products PEAK Matrix® Assessment 2024. The report assessed 13 providers based on the market impact of their products and their ability to deliver successful, high-quality offerings.
By Medidata · Via Business Wire · November 13, 2024

Medidata, a Dassault Systèmes brand and leader of clinical trial solutions to the life sciences industry, is kicking off NEXT New York 2024, its premier life sciences conference. Uniting more than 1,000 healthcare customers and partners, the event will showcase AI-powered innovations that accelerate clinical trials, streamline data management, and enhance decision-making for life-saving therapies.
By Medidata · Via Business Wire · November 13, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced a new enterprise agreement with Bioforum, a biometrics CRO that serves clinical trial sponsors worldwide.
By Medidata · Via Business Wire · November 12, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, has introduced two new bundled offerings to meet the growing demands of oncology and vaccine research. Medidata Oncology Solutions and Medidata Vaccine Solutions reinforce the FDA guidance for patient-centered endpoints, adaptive trial designs, and trial diversity. By unifying key trial components such as real-time patient-reported outcomes and imaging management, these bundled solutions will aid sponsors by reducing trial complexity, accelerating decision-making, and improving assessments of treatment efficacy and safety.
By Medidata · Via Business Wire · October 31, 2024

Medidata, a Dassault Systèmes brand and the leading provider of clinical trial solutions to the life sciences industry, today announced a partnership with neuroscience solutions leader Cogstate to reshape clinical trials and outcomes measurement for central nervous system (CNS) diseases across neurodegenerative, psychiatric, motor, and rare neurodevelopmental disorders, among others.
By Medidata · Via Business Wire · October 29, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced Medidata Rave Lite, an extension of the company’s gold-standard clinical research software, Medidata Rave EDC, designed explicitly for Phase I and Phase IV studies. Regardless of company size, therapeutic focus, or pipeline, Rave Lite provides efficient electronic clinical data capture (EDC), management, and analysis solutions with a tailored pricing model.
By Medidata · Via Business Wire · October 17, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced the launch of Medidata Patient Payments, a new solution that streamlines trial-related stipends and reimbursements for patients participating in clinical research. This offering automates the payment lifecycle and addresses the longstanding challenge of compensating participants for their time, effort, and study-related expenses, while solving for growing concerns around the financial toxicity of clinical trial encounters.
By Medidata · Via Business Wire · September 25, 2024

Medidata, a Dassault Systèmes brand and the leading provider of clinical trial solutions to the life sciences industry, has been recognized as a Leader in Everest Group‘s first-ever PEAK Matrix® Assessment for Electronic Data Capture (EDC) in life sciences.
By Medidata · Via Business Wire · September 17, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, and the European Organisation for Research and Treatment of Cancer (EORTC), announced today a four-year extension to their partnership. The extended relationship will enable EORTC to further increase patient access to oncology clinical trials, make trial participation easier, and help to deliver new treatments to the market faster. EORTC is now leveraging 13 Medidata solutions, enabling their researchers to access and manage all clinical data in a single place and offering patients a seamless trial experience.
By Medidata · Via Business Wire · September 11, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced a new project with Aixial Group, a contract research organization (CRO), to streamline the execution of studies and achieve database lock faster. Aixial Group will migrate clinical data from one of its business units onto the Medidata Platform to create a Single Source of Truth (SSOT) that enables study teams to support patient data privacy, monitor throughout the study to keep patients safe, and make better-informed decisions.
By Medidata · Via Business Wire · September 3, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced Eisai Inc. (“Eisai”), the U.S. pharmaceutical subsidiary of Tokyo-based Eisai Co., Ltd., as one of the first customers to harness its recently announced AI-driven Medidata Clinical Data Studio. Eisai Inc., will leverage this innovative data experience to gain unprecedented control over its clinical data, enable the execution of scalable and complex clinical trials, and enhance patient experience.
By Medidata · Via Business Wire · July 26, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced the launch of Medidata Clinical Data Studio, a unified experience that unlocks the true power of clinical research data. This groundbreaking technology gives stakeholders greater control over the quality of data and the ability to deliver safer trials to patients faster.
By Medidata · Via Business Wire · June 18, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, announced today that it has been selected by Lexicon Pharmaceuticals, Inc. to help advance PROGRESS, a Phase 2b study of LX9211 in diabetic peripheral neuropathic pain (DPNP), with the potential for LX9211 to become the first new, non-opioid drug approved for neuropathic pain in over two decades. Medidata will enable Lexicon to accelerate patient enrollment and clinical trials for Lexicon’s AAK1 inhibitor LX9211, improving the patient experience with a focus on addressing the high, unmet need for chronic neuropathic pain therapies.
By Medidata · Via Business Wire · May 29, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, today announced an expansion of its relationship with Worldwide Clinical Trials (“Worldwide”), a full-service global contract research organization (CRO). The agreement will facilitate data-driven decision-making across Worldwide’s entire clinical operations with the implementation of Medidata AI and enable Worldwide to support customers by reducing trial timelines and improving site selection.
By Medidata · Via Business Wire · May 14, 2024

Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, was presented with the inaugural Site Innovation Award for its groundbreaking work in improving efficiency in clinical trials by the Summit for Clinical Ops Executives (SCOPE).
By Medidata · Via Business Wire · March 26, 2024

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, today announced it has renewed its relationship with the PPD clinical research business of Thermo Fisher Scientific Inc.
By Medidata · Via Business Wire · February 28, 2024

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, and Sanofi Vaccines, today announced a collaboration to harness Medidata eCOA to deploy in vaccine studies. This builds on Medidata and Sanofi’s longstanding, successful experience using Medidata Rave EDC (electronic data capture).
By Medidata · Via Business Wire · February 6, 2024

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, has again been recognized as a Leader in Everest Group’s recently published “Decentralized Clinical Trials Platforms PEAK Matrix® Assessment 2023.” Medidata was the only provider to receive a “Star Performer” designation for the second consecutive year, reflecting its continued innovation and leadership.
By Medidata · Via Business Wire · January 10, 2024

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, today announced the launch of the Medidata Research Alliance. This unique consortium brings together leading clinician-researchers and key opinion leaders from academia, non-profit organizations, and the life sciences industry to leverage Medidata’s expertise in Artificial Intelligence (AI) capabilities and clinical trial data to drive cutting-edge research into innovative treatments.
By Medidata · Via Business Wire · December 7, 2023

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, today announced new experiences for enhancing and streamlining clinical data workflows: Clinical Data Studio and Health Record Connect. These solutions and others are being previewed at Medidata NEXT New York 2023, the premier clinical trials conference taking place from November 7-8 in New York City.
By Medidata · Via Business Wire · November 8, 2023

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, will host NEXT New York 2023, the premier clinical research conference taking place in New York City from November 7- 8. The gathering of more than 700 life sciences leaders will provide a platform for attendees to experience groundbreaking innovations that push the boundaries of the life sciences value chain — from research to commercialization.
By Medidata · Via Business Wire · November 2, 2023

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, today announced a multi-year partnership expansion with Catalyst Clinical Research to support their global oncology brand, Catalyst Oncology. This renewal builds upon Catalyst’s existing success with the Medidata Platform and incorporates wider offerings, including Medidata Grants Manager and Medidata AI Intelligent Trials, as Catalyst has grown into a robust and global full-service clinical research organization.
By Medidata · Via Business Wire · October 26, 2023

Medidata, a Dassault Systèmes company and leading provider of clinical trial solutions to the life sciences industry, today announced an extension of its 15-year relationship with the National Cancer Institute (NCI), part of the National Institutes of Health. This renewed commitment, spanning an additional five years, consolidates their joint efforts and underscores their dedication to advancing cancer research.
By Medidata · Via Business Wire · October 4, 2023

Medidata, a Dassault Systèmes company, today announced the launch of the Medidata Diversity Program, the industry’s most comprehensive solution for improving diversity, equity, and inclusion in clinical trials.
By Medidata · Via Business Wire · September 19, 2023

Medidata, a Dassault Systèmes company, was rated the pharmaceutical industry’s preferred provider of electronic data capture (EDC) solutions in a new report by Industry Standard Research. This report also identified Medidata’s Rave EDC as the No. 1 most recently used EDC system across the industry.
By Medidata · Via Business Wire · September 13, 2023

Medidata, a Dassault Systèmes company, today announced that Launch Therapeutics selected Medidata AI Intelligent Trials to support its innovative approach to accelerating clinical trials of late-stage therapies. Launch Therapeutics (Launch Tx) is a clinical development company with a mission to disrupt the late-stage development paradigm, accelerate timelines to regulatory success, and bring new medicines to patients faster.
By Medidata · Via Business Wire · April 12, 2023

Medidata, a Dassault Systèmes company, is enabling Intrials, a Latin American contract research organization (CRO), to expand its business with Medidata’s Rave CTMS, Rave eTMF, and Rave Site Payments. The agreement, which includes training and support from Medidata Professional Services, helps Intrials advance its offerings in Latin America with the capability to manage more complex trials and attract larger global clients.
By Medidata · Via Business Wire · June 28, 2023

Medidata, a Dassault Systèmes company, honored life sciences industry innovators and two inspirational leaders during its NEXT conference. A highly anticipated feature at the event, the Medidata NEXT Awards recognize excellence in collaboration, sustainability, digital innovation, and patient leadership.
By Medidata · Via Business Wire · March 30, 2023

Medidata, a Dassault Systèmes company, launched Rave EDC Certified Study Builder certification, a new offering in its global education program for study build and study management professionals. The Rave EDC Certified Study Builder Program offers a guided learning path to certification for new and existing Rave study builders, and includes core skills and applied skills assessments.
By Medidata · Via Business Wire · March 23, 2023

Medidata, a Dassault Systèmes company, today announced that Allucent, a contract research organization (CRO) bringing innovation to biopharma companies, is expanding the global use of Medidata’s Clinical Cloud to advance its end-to-end clinical trial operations offering.
By Medidata · Via Business Wire · March 7, 2023

Known for its ground-breaking technological innovations in clinical trials, Medidata, a Dassault Systèmes company, has exceeded 30,000 clinical trials and 9 million study participants. These milestones, achieved together with more than 2,100 global customers and partners, have been fueled by Medidata’s platform and life sciences solutions that power smarter treatments and healthier people, while driving greater access to trials.
By Medidata · Via Business Wire · March 2, 2023

Medidata, a Dassault Systèmes company, today announced a new leadership structure designed to fuel its mission of creating end-to-end solutions that power smarter treatments and healthier people. The strategic evolution is an opportunity to further long-term goals and growth to enhance customers’ ability to unlock greater value across the Dassault Systèmes portfolio, ultimately taking drug development to new heights.
By Medidata · Via Business Wire · February 21, 2023

Medidata, a Dassault Systèmes company, announced it has been positioned as a Leader in the IDC MarketScape “Worldwide Life Science R&D Risk-Based Monitoring Solutions 2022 Vendor Assessment (doc #US48061722).” The IDC MarketScape assessment evaluated Medidata’s comprehensive RBQM (Risk-Based Quality Management) capabilities including Medidata Detect, which provides clinical operations teams with the ability to proactively monitor and mitigate risks to data integrity and patient safety.
By Medidata · Via Business Wire · February 6, 2023

Medidata, a Dassault Systèmes company, will be leading multiple sessions at the upcoming Summit for Clinical Ops Executives (SCOPE) on trial diversity, risk based quality management (RBQM), decentralized clinical trials (DCT), real world data, sensors and wearables, and randomization and trial supply management (RTSM). Medidata is a top signature sponsor of the event held February 6-9.
By Medidata · Via Business Wire · February 3, 2023

Medidata, a Dassault Systèmes company, has been named a Leader and Star Performer in the “Decentralized Clinical Trial Platforms PEAK Matrix Assessment 2023” by Everest Group. The annual report evaluates decentralized clinical trial (DCT) products from 24 companies based on vision, capability, and market impact.
By Medidata · Via Business Wire · December 13, 2022

Medidata, a Dassault Systèmes company, has been honored by the Reagan-Udall Foundation for the Food and Drug Administration (FDA) with its 2022 Innovation in Regulatory Science Award for Medidata Link. This technology breakthrough, based on Medidata’s Rave EDC (electronic data capture) allows sponsors and regulators to connect and analyze once-disparate data sources to bridge the gap between the clinical trial and real world information sources.
By Medidata · Via Business Wire · December 8, 2022

Medidata, a Dassault Systèmes company, launched a new patient-centric native myMedidata app, designed to provide trial participants with another option for a seamless platform experience and a single login for all their remote trial activities. The app will feature all of the myMedidata patient-centric solutions, with an initial focus on eCOA (electronic clinical outcome assessments). The myMedidata app is available on iOS and Android and can be used both on the patients’ own devices (BYOD) or via a provisioned device.
By Medidata · Via Business Wire · December 6, 2022

Medidata, a Dassault Systèmes company, announced that vice president for Patient Engagement and leader of its Patient Insights Board Alicia Staley has been honored with the Ellen L. Stovall Award for patient advocacy from the National Coalition for Cancer Survivorship (NCCS).
By Medidata · Via Business Wire · November 22, 2022

Medidata, a Dassault Systèmes company, today announced plans to launch Rave Companion, an innovative, scalable, patent pending technology helping clinical trial sites save time and reduce errors in transferring EHR (electronic health record) data to the Rave EDC (electronic data capture) system. Rave Companion addresses the long-standing industry challenge of duplicate data entry by enabling structured and unstructured data from any electronic health record to be directly utilized by Rave EDC, within a few clicks.
By Medidata · Via Business Wire · November 15, 2022

Medidata, a Dassault Systèmes company, will gather hundreds of the best and brightest members of the life sciences industry to explore the latest innovations—from the next generation of clinical data management to scaling decentralized trials through advanced analytics and patient insights.
By Medidata · Via Business Wire · November 7, 2022

Medidata, a Dassault Systèmes company, and Boehringer Ingelheim today announced a five-year renewal of their collaboration in the wider area of electronic data capture. The new agreement extends the use of Rave EDC for Boehringer Ingelheim's clinical trials worldwide and includes myMedidata, Medidata’s suite of innovative patient-facing technologies focused on enhancing patient centricity and diversity in decentralized clinical trials (DCTs).
By Medidata · Via Business Wire · October 27, 2022

Medidata, a Dassault Systèmes company, has launched the Intelligent Trials Diversity Module to help improve the equity of clinical trials by providing site-level participant demographic data including race, sex, age, and ethnicity. The new Intelligent Trials Diversity Module will help sponsors and clinical research organizations (CROs) benchmark the diversity of their trials and identify sites that are more successful at enrolling diverse patients. This insight will help bring diversity into the beginning of the feasibility process, all while accelerating trials.
By Medidata · Via Business Wire · October 13, 2022

Experts from Medidata, a Dassault Systèmes company, will be chairing sessions on promoting consensus between regulators and the industry, imaging quality control, and presenting a product showcase on Medidata Clinical Cloud at the Society for Clinical Data Management (SCDM) annual conference. Medidata innovators will also be participating in multiple presentations and panels covering a range of innovative solutions to support the evolution from Clinical Data Management (CDM) to Clinical Data Science excellence. Medidata is a platinum sponsor of this year’s conference, which is being held in San Antonio, September 11 - 14.
By Medidata · Via Business Wire · September 7, 2022

Medidata, a Dassault Systèmes company, is supporting the White House Cancer Moonshot initiative by working with the National Cancer Institute (NCI) to enroll patients in the Cancer Moonshot Biobank. The Biobank is a project designed to better understand how cancer develops and grows, and why some cancers stop responding to treatment. People with cancer are asked to donate tumor tissue, blood samples, and health data to find discoveries that improve cancer treatments and increase survival.
By Medidata · Via Business Wire · July 27, 2022

Medidata, a Dassault Systèmes company, announced 10 highly innovative organizations have joined its groundbreaking Sensor Cloud Network. AliveCor, Aural Analytics, Biobeat, Blue Spark Technologies, Glooko, Indie Health, University of Arizona, Carnegie Mellon University, University of Rochester, and University of Vermont are now part of the first cross-sector collaboration focused on solving the challenges related to sensor integrations, standardization of sensor data, and the development of novel digital biomarkers and algorithms. These will help to create new digital endpoints that could translate into more effective treatments and better healthcare for patients.
By Medidata · Via Business Wire · June 29, 2022

Medidata, a Dassault Systèmes company, announced technology enhancements that address key issues in clinical trial management and oversight. Enhancements to Medidata Detect and Rave CTMS (Clinical Trial Management System) will improve both data oversight and reporting for sponsors and contract research organizations (CROs) in two meaningful ways: how they comprehensively monitor their trial data, and how they visualize those data to make faster decisions.
By Medidata · Via Business Wire · June 27, 2022

Medidata, a Dassault Systèmes company, will be leading an innovative educational workshop on how to master decentralized clinical trials (DCTs), sessions on risk-based monitoring and adaptive designs, and more as a premium sponsor of the DIA (Drug Information Association) Global Annual Meeting, June 19-23. Medidata experts will also be participating in multiple presentations and panels covering a range of innovative solutions to advance clinical trial planning, management, and operations.
By Medidata · Via Business Wire · June 15, 2022

Medidata, a Dassault Systèmes company, presented new research on predicting cytokine release syndrome (CRS) resulting from longitudinal CAR T-cell therapy (CAR T) at the American Society for Clinical Oncology (ASCO) Annual Meeting being held June 3-7 in Chicago.
By Medidata · Via Business Wire · June 3, 2022

Medidata, a Dassault Systèmes company, announced that Ergomed’s Rare Disease Innovation CenterTM will leverage Medidata’s Patient Cloud, Intelligent Trials technologies, and Synthetic Control ArmⓇ to help sponsors reduce clinical trial timelines, optimize patient experiences, and bring effective rare disease therapies to market more quickly.
By Medidata · Via Business Wire · May 5, 2022

Medidata, a Dassault Systèmes company, announced today that it has won a Forrester Program of the Year Award for successfully implementing Forrester’s research, frameworks, and best practices to change its approach to market planning, including audience-centric campaigns. Program of the Year (PoY) Award winners are being recognized at the B2B Summit North America, being held in Austin, Texas, and digitally, May 2–4, 2022.
By Medidata · Via Business Wire · May 2, 2022

Medidata, a Dassault Systèmes company, is pleased to announce that it was recognized for its outstanding accomplishments in life sciences by the Citeline Awards:
By Medidata · Via Business Wire · April 11, 2022

Medidata, a Dassault Systèmes company, announced that Labcorp, a leading global life sciences company, has selected Medidata's technology platform to extend their 14-year partnership, the foundation of their initiative to co-develop digital biomarkers and expand the use and functionality of decentralized clinical trials.
By Medidata · Via Business Wire · March 31, 2022

Medidata, a Dassault Systèmes company, today announced it is allowing sponsors, CROs (contract research organizations), and sites to leverage its proprietary Patient Centricity by Design process for clinical trials. Medidata will open access to its Patient Insights Program as part of a new offering to incorporate a patient-centric approach to the design and development of clinical trial protocols and technologies.
By Medidata · Via Business Wire · February 15, 2022

Medidata, a Dassault Systèmes company, announced today that Parexel, a leading global clinical research organization (CRO) focused on development and delivery of innovative new therapies to advance patient health, is extending their 15-year global strategic partnership. This builds upon the trusted relationship between the two companies and sets the goal of jointly pioneering a new era of decentralized clinical trial technology (DCT) for the life sciences industry.
By Medidata · Via Business Wire · February 4, 2022

Medidata, a Dassault Systèmes company, will be sharing insights into new decentralized clinical trials (DCTs) and AI technologies as a signature sponsor of the annual Summit for Clinical Ops Executives (SCOPE), February 7-10. Medidata experts will be participating in multiple presentations covering a range of innovative solutions to advance clinical trial planning, management, and operations.
By Medidata · Via Business Wire · February 3, 2022

Medidata, a Dassault Systèmes company, today announced a groundbreaking and exclusive partnership with Circuit Clinical, an Integrated Research Organization (IRO). Circuit Clinical has created a network representing over 90 doctors, across 30+ site locations, and a nationally accredited cancer center with a database of more than 2.5 million participants who may qualify for clinical trials. With Medidata’s strategic investment in Circuit Clinical's $27M Series C funding, Circuit Clinical will expand its Decentralized Clinical Trial (DCT) network to 500 physician investigators and 15M patients and help to bring clinical research as a care option to more people in need.
By Medidata · Via Business Wire · January 26, 2022

Medidata, a Dassault Systèmes company, today announced that Translational Drug Development (TD2), a precision oncology contract research organization (CRO), has signed an agreement to adopt Medidata’s Rave CTMS (Clinical Trial Management System) and eTMF (electronic Trial Master File) solutions. Rave CTMS and eTMF are key offerings within Medidata’s Unified Platform, a cutting-edge platform that is transforming the clinical trial experience for patients, sponsors, CROs, and research sites.
By Medidata · Via Business Wire · October 14, 2021

Medidata, a Dassault Systèmes company, today announced that Rave Imaging, the company’s cloud-based, secure clinical trial imaging management platform, has reached a significant milestone, having supported more than 1,000 imaging studies. Rave Imaging, built on the Medidata Unified Platform, processes more than 100 million images annually. The technology provides unprecedented real-time visibility into all imaging-related trial activities across all Rave Imaging trials to enhance study efficiency.
By Medidata · Via Business Wire · October 12, 2021

Medidata, a Dassault Systèmes company, today announced the launch of Medidata Link at NEXT Global 2021. Medidata Link is the only centralized solution to connect patient-level clinical trial data and real-world data (RWD) – powered by and fully integrated with the Medidata Unified Platform – providing any clinical trial run on Medidata Rave EDC (electronic data capture) the option to conduct data linkage.
By Medidata · Via Business Wire · October 7, 2021

Medidata, a Dassault Systèmes company, today announced the launch of Medidata Sensor Cloud Network at NEXT Global 2021. The Medidata Sensor Cloud Network will create the first industry-wide collaboration amongst contract research organizations (CROs), device manufacturers, drug and vaccine developers, analytics companies, and academia focused on solving challenges related to sensor integrations, standardization of sensor data, and the development of digital biomarkers and algorithms. This collaborative environment and approach is unique in its ability to connect to the Medidata Sensor Cloud, which provides the ability to ingest, normalize, and analyze data into a common format.
By Medidata · Via Business Wire · October 6, 2021

Medidata, a Dassault Systèmes company, today announced that Rho, a full-service contract research organization (CRO) with a proven track record of drug development success, is using Medidata to standardize its clinical trial platform for building out its decentralized clinical trial (DCT) technology offerings. This will enable Rho to provide rapid reporting of cross-domain, cross-study data in decentralized trials, where data collection methods have become more numerous, varied, and complex.
By Medidata · Via Business Wire · October 5, 2021

Medidata, a Dassault Systèmes company will convene experts in drug and device development, clinical operations, data management, biostatistics, and digital medicine industries to help solve the impossible at NEXT Global from October 5-7, 2021. The industry’s largest meeting of its kind includes renowned keynote speakers, live and interactive roundtable discussions, breakout sessions, lightning talks, customer success stories, product demonstrations, networking, and wellness activities.
By Medidata · Via Business Wire · September 28, 2021

Medidata, a Dassault Systèmes company, recently named Rama Kondru and Sastry Chilukuri co-CEOs. Both are Medidata veterans, with Chilukuri as the founding president of Medidata Acorn AI and Kondru as executive vice president and chief technology officer. Collaborating as CEOs will be nothing new for these industry leaders, having also partnered in roles prior to joining Medidata.
By Medidata · Via Business Wire · September 27, 2021

Medidata, a Dassault Systèmes company, and Labcorp Drug Development, a global company providing clinical laboratory and end-to-end drug development services, today announced they have entered into an agreement using the Medidata Sensor Cloud. Medidata will receive and process medical-grade sensor data within drug, vaccine and device trials across Labcorp Drug Development’s clinical trial portfolio, enhancing their decentralized clinical trial (DCT) offerings.
By Medidata · Via Business Wire · September 13, 2021

Medidata, a Dassault Systèmes company, today announced a significant enhancement of myMedidata eConsent that enables video visits with patients and study staff. With video eConsent, trial participants can engage in remote, face-to-face, live conversation between patients and clinical trial site staff during the informed consent process. Building upon existing eConsent functionality, video eConsent represents yet another step in the revolutionary changes being brought about by decentralized clinical trial (DCT) technology. This expansion of the company’s telemedicine/video visit capability is the most recent addition to the myMedidata portal designed to be a single patient platform for all patient-centered technology.
By Medidata · Via Business Wire · August 17, 2021

Medidata, a Dassault Systemes company, today announced that the Medidata Acorn AI Synthetic Control Arm® (SCA) has been awarded “Best AI-based Solution for Healthcare'' in this year’s AI Breakthrough Awards.
By Medidata · Via Business Wire · June 30, 2021

“Tu Salud Tu Familia,” (Your Family Your Health) a weekly health and medical education program on Telemundo Washington, D.C., won the technology category at the 63rd Capital Emmy® Awards presented by the National Capital Chesapeake Bay Chapter of The National Academy of Television Arts and Sciences. This is the second Capital Emmy win for the show.
By Medidata · Via Business Wire · June 28, 2021
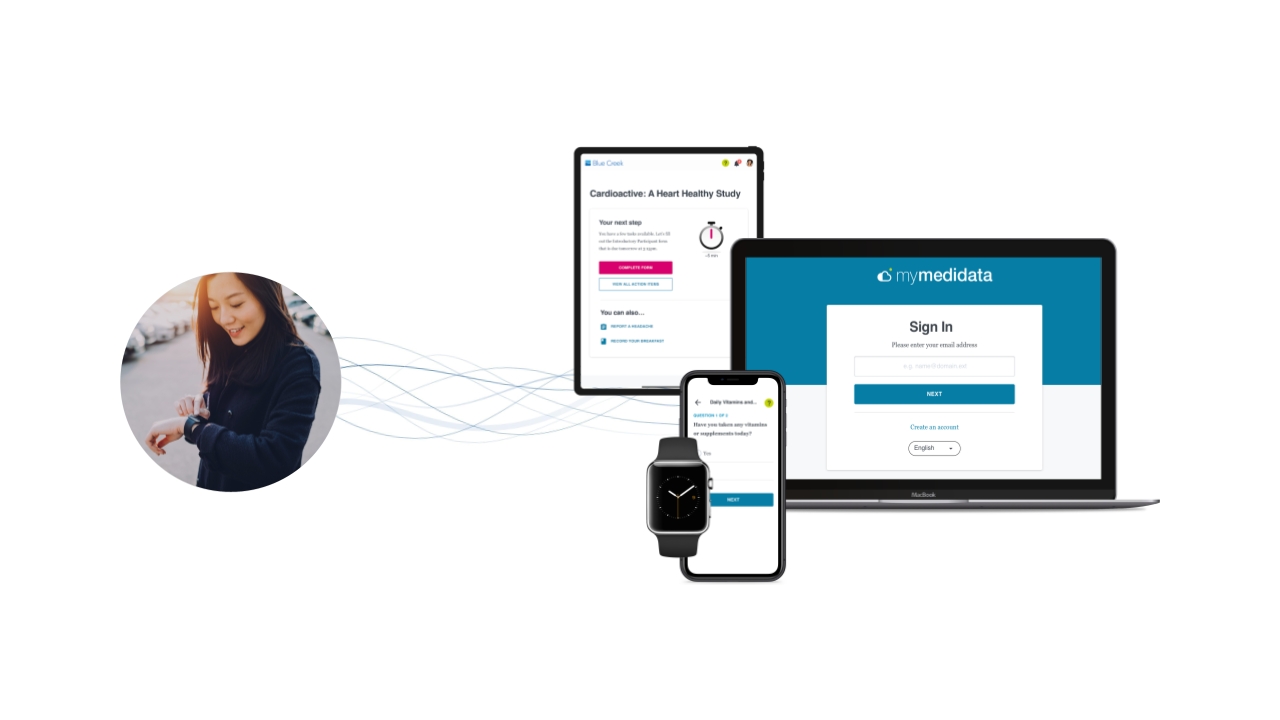
Medidata, a Dassault Systèmes company, today announced the launch of the Medidata Decentralized Clinical Trials (DCT) Program, the most comprehensive set of unified, secure technologies that enable full decentralization across the clinical trial continuum. For the first time ever, drug, vaccine, and medical device developers (sponsors) and contract research organizations (CROs) can take advantage of the only platform offering on the market which combines:
By Medidata · Via Business Wire · June 15, 2021
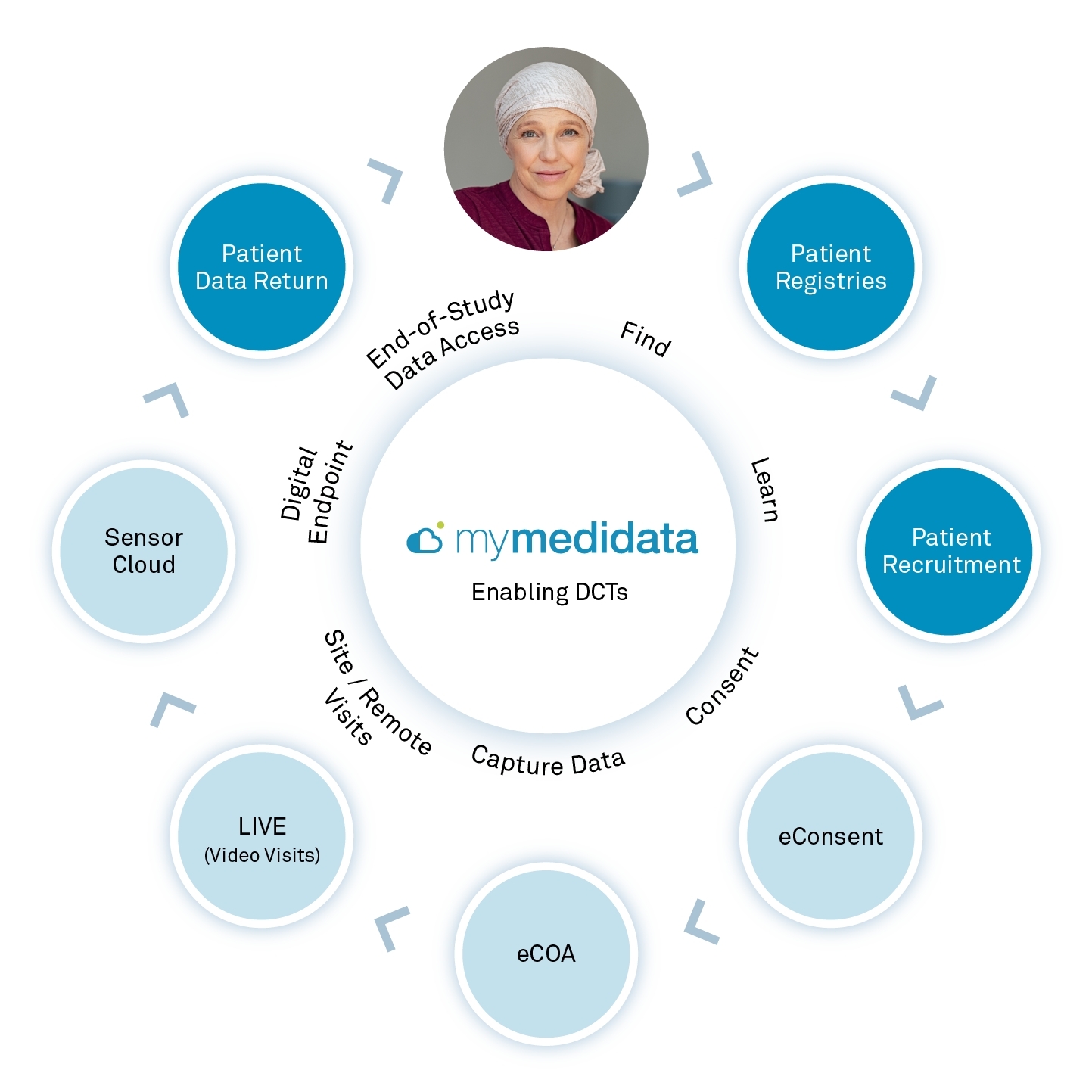
Medidata, a Dassault Systèmes company, today launched myMedidata Registries, a new technology that expands and strengthens the myMedidata patient portal to engage patients before and after (i.e., long term follow up/safety surveillance) a clinical trial. This empowers patients to learn more about clinical trial opportunities and provides an experience that allows for active participation throughout their clinical trial journey. With increasing interest and adoption of decentralized clinical trials, myMedidata Registries gives patients continuous support in and out of a trial with access to one portal for all of their research needs - providing an everlasting engagement on one portal for life.
By Medidata · Via Business Wire · June 8, 2021

Medidata, a Dassault Systèmes company, the global leader in creating end-to-end solutions supporting the entire clinical trial process, today announced that its myMedidata solution has been selected as the winner of the ‘Best Patient Portal’ award in the fifth annual MedTech Breakthrough Awards program conducted by MedTech Breakthrough, an independent market intelligence organization that recognizes the top companies, technologies and products in the global health and medical technology market.
By Medidata · Via Business Wire · May 6, 2021
