Immunovant, Inc. - Common Stock (IMVT)
26.00
-0.45 (-1.70%)
NASDAQ · Last Trade: Feb 1st, 9:38 PM EST
Detailed Quote
| Previous Close | 26.45 |
|---|---|
| Open | 26.20 |
| Bid | 25.18 |
| Ask | 27.00 |
| Day's Range | 25.42 - 26.42 |
| 52 Week Range | 12.72 - 27.80 |
| Volume | 1,825,472 |
| Market Cap | 2.99B |
| PE Ratio (TTM) | -9.187 |
| EPS (TTM) | -2.8 |
| Dividend & Yield | N/A (N/A) |
| 1 Month Average Volume | 1,523,118 |
Chart
About Immunovant, Inc. - Common Stock (IMVT)
Immunovant Inc. is a clinical-stage biopharmaceutical company focused on developing innovative therapies for patients with autoimmune diseases. Utilizing its proprietary technology, Immunovant aims to create monoclonal antibodies that target specific pathways involved in these conditions, with the goal of providing safer and more effective treatment options. The company's research and development efforts are centered on addressing unmet medical needs, improving patient outcomes, and enhancing the quality of life for individuals suffering from autoimmune disorders. Through its commitment to scientific advancement and patient care, Immunovant actively works to bring novel treatments to the market. Read More
News & Press Releases

Focused on monoclonal antibody therapies for autoimmune diseases, this biotech just reported a significant insider buy in public filings.
Via The Motley Fool · December 18, 2025

Immunovant stock drops 10% despite meeting Q2 FY2026 earnings estimates. The sell-off is driven by investor reaction to clinical pipeline updates for its anti-FcRn therapies.
Via Chartmill · November 10, 2025

Via Benzinga · November 10, 2025

Via Benzinga · October 30, 2025

NEW YORK and NEW ORLEANS, Sept. 17, 2025 (GLOBE NEWSWIRE) -- The law firm of Kahn Swick & Foti, LLC (“KSF”) has commenced an investigation into Immunovant, Inc. (NasdaqGS: IMVT) (“Immunovant”). KSF is investigating whether Immunovant’s officers and/or directors breached their fiduciary duties or otherwise violated state or federal laws.
By Kahn Swick & Foti, LLC · Via GlobeNewswire · September 17, 2025

The law firm of Kahn Swick & Foti, LLC (“KSF”) has commenced an investigation into Immunovant, Inc. (NasdaqGS: IMVT) (“Immunovant”). KSF is investigating whether Immunovant’s officers and/or directors breached their fiduciary duties or otherwise violated state or federal laws.
By Kahn Swick & Foti, LLC · Via Business Wire · September 12, 2025

The biotech looks as if it has a promising treatment for an autoimmune disorder.
Via The Motley Fool · September 3, 2025

The Schall Law Firm, a national shareholder rights litigation firm, announces that it is investigating claims on behalf of investors of Immunovant, Inc. (“Immunovant” or “the Company”) (NASDAQ: IMVT) for violations of the securities laws.
By The Schall Law Firm · Via Business Wire · August 27, 2025

The law firm of Kahn Swick & Foti, LLC (“KSF”) has commenced an investigation into Immunovant, Inc. (NasdaqGS: IMVT) (“Immunovant”). KSF is investigating whether Immunovant’s officers and/or directors breached their fiduciary duties or otherwise violated state or federal laws.
By Kahn Swick & Foti, LLC · Via Business Wire · August 13, 2025

PITTSBURGH, PA / ACCESS Newswire / June 20, 2025 / Law Office of Alfred G. Yates Jr. PC announces investigation into potential breaches of fiduciary duty by the directors and officers of Immunovant, Inc. (NASDAQ:IMVT) and Roivant Sciences Ltd. - Immunovant's controlling stockholder - in connection with Immunovant's January 2025 private placement transaction in which Roivant purchased nearly 16.9 million Immunovant shares at a price of $20.00 per share, a $3.48 per share discount to the prior trading day's closing price.
Via ACCESS Newswire · June 20, 2025

The clinical-stage immunology company reported a net loss of $0.64 per share for the quarter, lower than an estimated loss of $0.72 per share.
Via Stocktwits · May 29, 2025

Robbins Geller Rudman & Dowd LLP announces an investigation into potential breaches of fiduciary duty by the directors and officers of Immunovant, Inc. (NASDAQ: IMVT) and Roivant Sciences Ltd. – Immunovant’s controlling stockholder – in connection with Immunovant’s January 2025 private placement transaction in which Roivant purchased nearly 16.9 million Immunovant shares at a price of $20.00 per share, a $3.48 per share discount to the prior trading day’s closing price.
By Robbins Geller Rudman & Dowd LLP · Via Business Wire · May 21, 2025

Via Benzinga · April 22, 2025

The company is getting a new chief executive and a new chief financial officer amid sweeping changes.
Via Investor's Business Daily · April 21, 2025
Immunovant stated that these changes are part of its parent entity, Roivant, increasing operational involvement and strategic oversight of the company.
Via Stocktwits · April 21, 2025

NEW YORK, April 21, 2025 (GLOBE NEWSWIRE) -- Immunovant, Inc. (Nasdaq: IMVT), a clinical-stage immunology company dedicated to enabling normal lives for people with autoimmune diseases, today announced next phase of growth including changes to its leadership team and the expanded development of IMVT-1402 into two new indications, SjD and CLE.
By Roivant Sciences · Via GlobeNewswire · April 21, 2025

Immunovant's batoclimab showed positive Phase 3 results in MG but won't be pursued for approval as the company shifts focus to its lead asset, IMVT-1402.
Via Benzinga · March 19, 2025

The companies reported positive results for their lead asset in patients with two rare diseases.
Via Investor's Business Daily · March 19, 2025

Via Benzinga · March 19, 2025

Analysts remains bullish on the company's chances in depression and epilepsy. But investors aren't convinced.
Via Investor's Business Daily · March 4, 2025
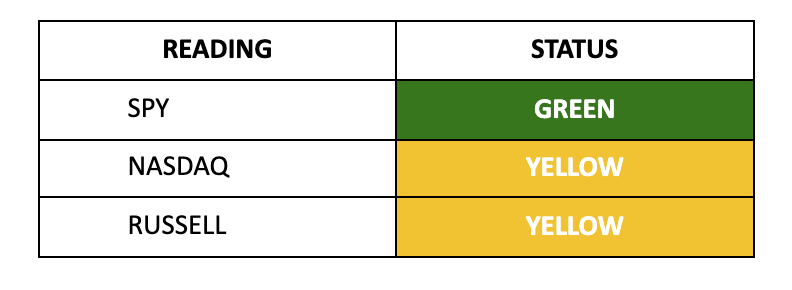
With inflation and jobs behind us, it’s time to shift our focus 6,700 miles west to Tokyo. The Bank of Japan appears poised for another rate hike next week, with odds now at 77%. Monitor the VIX for early warning signals.
Via Talk Markets · January 19, 2025

Amgen tested its drug, Uplizna, in generalized myasthenia gravis, a market where Argenx's Vyvgart leads.
Via Investor's Business Daily · September 25, 2024



